
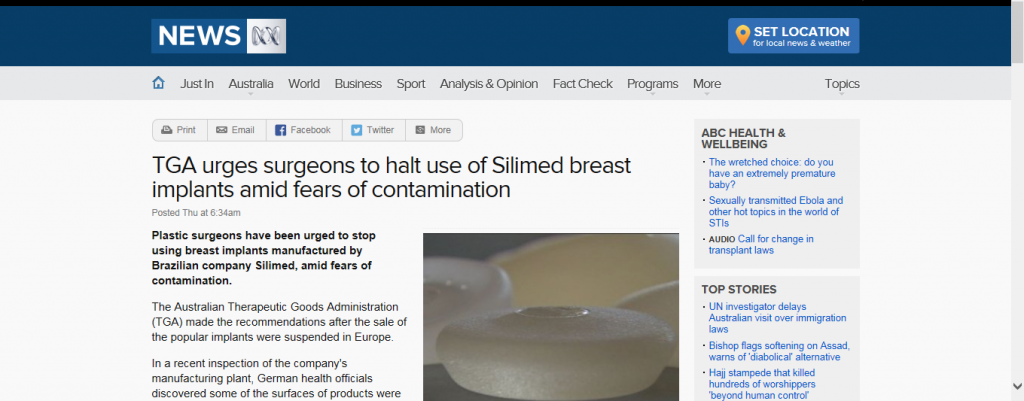
In the past, the FDA has cornered the market on raising safety and viability issues with the breast implant industry taking silicone breast implants completely off the market from 1991 – 2006! Yesterday it’s European equivalent, The Medicines and Healthcare products Regulatory Agency (MHRA), took center stage in the world of breast implant controversy by suspending the CE mark for Silimed medical devices, including breast implants.
The Plastic Surgery Channel
William P. Adams, Jr. MD of Dallas, Texas, specializes in breast augmentation and is no stranger to breast implants and the controversies that seem to follow them. He was actually in Europe earlier this week speaking and teaching on the safety and efficacy of breast implants when this announcement was made. “The Silimed CE mark suspension of all company-wide medical devices made by MHRA, an executive agency of the United Kingdom Department of Health, was big news on Wednesday in Europe. The exact issue was unclear and length of the suspension unknown,” explains Dr. Adams.
Back across the pond, US based Sientra communicated to its many users as Silimed is one of Sientra’s contract manufacturers. The communication from CEO Hani Zeini specifically stated:

Although no true clinical issues have materialized to date, investors are certainly taking notice. Sientra’s stock dropped 50% yesterday.
Patient safety is paramount and given US patient’s past experience with breast implant scares, this announcement might seem a little disturbing however; Dr. Adams says, “I have used Sientra’s products since their release and am fully confident in their quality, and will continue to use for patients we feel they are ideally suited for.”













Facebook
Twitter
Instagram
YouTube
RSS