On March 21, 2017, a new update was released from the Food and Drug Administration regarding BIA-ALCL has the media buzzing with talk of serious illness and safety. Seemingly none of the talk, however, is giving patients much real information.
Dr. Bill Adams, Associate Clinical Professor, Department of Plastic Surgery at UT Southwestern, Board member for the American Society of Aesthetic Plastic Surgery, and ASPS/ ASAP BI-ALCL Taskforce member and Dr. Jamil Ahmad, Assistant Professor, Division of Plastic and Reconstructive Surgery at the University of Toronto, and Board member for the American Society of Aesthetic Plastic Surgery, have received a number of calls from their patients who are now worried by the news coverage and are searching for real answers. Breast Implant ALCL is not a new topic for these two surgeons, the plastic surgery community, breast implant industry or the FDA. In fact, research and studies have been going on for years now to provide scientific data, real solutions and treatments.
What is BIA-ALCL?
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare disease that appears to be associated with textured breast implants. Based on confirmed data and publications, there have only been 242 cases reported worldwide. Patients with smooth breast implants can rest easy knowing that this issue has never been associated with the type of implant they have.
The FDA tends to lag behind published literature and cutting-edge research, in general. However, this is the first time that the FDA has associated BIA-ALCL with a particular breast implant – a textured implant. Most breast implants used in the United States are smooth – not textured. Studies show that textured implants tend to have a higher risk of BIA-ALCL because they offer a “medium” – more surface area for a bacteria to grow in and around. This type of environment promotes chronic inflammation.
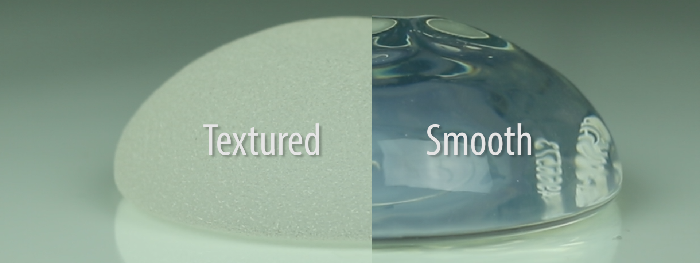
There are many different types of textured implants – both micro and macro-textured surfaces. The Australian equivalent of the FDA also recently issued an update on this topic stating that macro-textured implants were more likely to be involved in these cases than the less textured products. Patients with textured implants should not be alarmed but simply educated to signs and symptoms of change. If a patient with a textured implant notices a change in shape and size of their breasts, they should call their plastic surgeon and be seen for evaluation. In other words, how do you know if you might have a problem? A significant change in shape and size of your breasts is the tale tell sign.
Is There a Relation to Silicone?
Most patients remember the breast implant scare of the 90’s. It was a time of uncertainty for breast implant patients, mostly because of the lack of valid information. Everyone was scared that silicone was going to hurt them when in actuality, silicone was never proven to cause any of the horrible things the media said it did. The same is true now, BIA-ALCL is NOT related to silicone.

Dr. Ahmad asks a very important question on the minds of all breast implant patients reading this report, “Is BIA-ALCL a breast cancer?” Dr. Adams explains that although ALCL has been called cancer in the past, the risk of developing BIA-ALCL is much lower, estimated at 1 in 30,000. The current classification of BIA-ALCL was set by the WHO, however there is data to suggest that this may need to be changed as it behaves much more like a Lymphoproliferative Disorder. The FDA report suggests that there have been 9 deaths in the US over the past 55 years of breast implant history, although all of which had complicated histories with some patients incurring mortality secondary to chemotherapy and radiation treatment. All known cases of BIA-ALCL that were promptly diagnosed and treated have been cured.
Diagnosing and Treating BIA-ALCL
Typically, a patient would present with a swollen breast that is then aspirated and analyzed. If ALCL markers are present, the treatment and cure is to do a surgery where the capsule that surrounds the breast implant and the implant are removed. This is exactly how capsular contracture is treated as well. It is a common operation and has been shown to be extremely effective in the treatment of ALCL cases.
Current research shows that the plastic surgery community can use better intraoperative techniques to reduce not only ALCL risks but capsular contracture as well. The 14 Point Plan is a process that has been scientifically reviewed and proves to reduce the amount of bacteria around a breast implant thus reducing chronic inflammation and capsular contracture and ALCL risks.
The bottom line here is that patients should not be alarmed, but aware. Breast Implant-Associated ALCL is not new and there is excellent research and progress in reducing its occurrence and improving the treatment should it occur. Here’s what patients should know:
- It is a rare disease that appears to be associated with TEXTURED breast implants
- If you notice significant swelling or changes in breast shape and size, call your plastic surgeon
- If diagnosed and treated properly, it is curable
- Patients desiring breast implants should know the difference between textured and smooth implants and discuss the best option for them with their surgeon