Whenever a warning is raised about any type of cancer – even a rare one – and breast implants, it’s easy to strike fear and panic in patients. Typically, however, such announcements are far less grave than usual new involving cancer. The announcement just needs to be unpacked by surgeons and physicians in the know, put the issue in perspective and quell any unheeded concerns. This is certainly true with the latest news about certain breast implant patients and a type of lymphoma.
An FDA Warning that Prompted a Flood of Calls to Plastic Surgeons
Late last month, many news outlets, like CBS News, headlined the Food and Drug Administration announcement: “Nine deaths from rare cancer caused by breast implants.” The CBS story pointed out the link wasn’t new, but said there is a “risk to women with both silicone and saline breast implants.” That kind of blanket statement sent implant patients and prospective implant patients to speed dial their plastic surgeons.
Just What IS this Issue?
It’s a mouthful. Technically, it’s called Breast Implant-Associated Anaplastic Large Cell Lymphoma, or BIA-ALCL. Women diagnosed with the implant-linked cancer have reported pain, lumps, swelling or breast asymmetry. Dr. Maggie DiNome, a breast cancer surgeon and associate clinical professor of surgery at UCLA Medical Center, says women with implants should know that it’s a very rare form of cancer.
“They have only documented 300 or so cases of this. We’re talking about 300 out of 10 million women worldwide with implants, so it’s very, very rare and it’s very curable if you were to get it,” Dr. DiNome told CBS News, noting the implant would be taken out in most cases. She also cautioned that the symptoms of implant-linked ALCL, such as one breast being more swollen than the other, can be due to other issues with an implant not related to cancer, including infection or trauma. In cases not linked to breast implants, ALCL normally shows up on a blood test for the illness, typically ordered if a woman is experiencing enlarged lymph nodes, fatigue and other symptoms that prompt a physician to test for the condition.
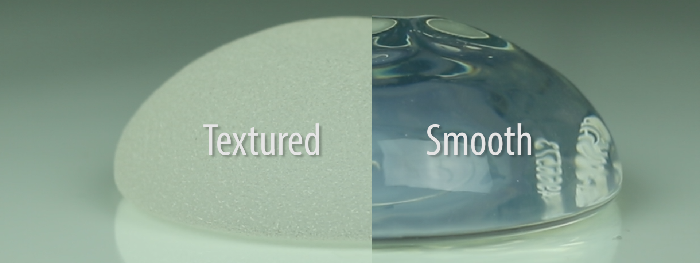
If you have Smooth Implants, this is Not Your Worry
Based on confirmed data and publications, there have only been 242 cases reported worldwide. Patients with smooth breast implants can rest easy knowing this issue has never been linked to this type of implant.
“The FDA tends to lag behind published literature and cutting-edge research, in general,” William P. Adams Jr., MD tells The Plastic Surgery Channel. “But this is the first time that the FDA has associated BIA-ALCL with a particular breast implant – textured ones. Most breast implants used in the United States are smooth – not textured. Studies show that textured implants tend to have a higher risk of BIA-ALCL because they offer a ‘medium’ – more surface area for a bacteria to grow in and around. This type of environment promotes chronic inflammation.”
There are many different types of textured implants – both micro and macro-textured surfaces. The Australian equivalent of the FDA also recently issued an update on this topic stating that macro-textured implants were more likely to be involved in these cases than the less textured products. Patients with textured implants should not be alarmed but simply educated to signs and symptoms of change. If a patient with a textured implant notices a change in shape and size of their breasts, they should call their plastic surgeon and be seen for evaluation. In other words, how do you know if you might have a problem? A significant change in shape and size of your breasts is the tale tell sign.
Is There Any Relation to Silicone?
Most patients remember the breast implant scare of the 90’s. It was a time of uncertainty for breast implant patients, mostly because of the lack of valid information. Everyone was scared that silicone was going to hurt them when in actuality, silicone was never proven to cause any of the horrible things the media said it did. The same is true now, BIA-ALCL is NOT related to silicone.
What is the FDA telling women to do?
The FDA said it will continue to collect information about the disease in women with breast implants and the agency encouraged health care providers to monitor implant patients regularly. They did not recommend implant removal in patients without symptoms or other abnormalities.
“If you already have breast implants, there is no need to change your routine medical care and follow-up,” the FDA statement said. It recommended that women who are considering implants weigh the risks and benefits with their health care providers.
Best Advice?
The bottom line: don’t be alarmed, just aware. Breast Implant-Associated ALCL is not new and there is excellent research and progress in reducing how often it occurs and what to do if it does.
The latest on BIA-ALCL:
- It is a rare disease that appears to be associated with textured breast implants
- If you notice significant swelling or changes in breast shape and size, call your plastic surgeon
- If diagnosed and treated properly, it is curable
- Patients desiring breast implants should know the difference between textured and smooth implants and discuss the best option for them with their surgeon















Facebook
Twitter
Instagram
YouTube
RSS