Dermal fillers are a popular treatment to minimize wrinkles and eliminate the signs of aging. Now, the FDA has approved a new version of a popular filler premixed with lidocaine to take away the sting.
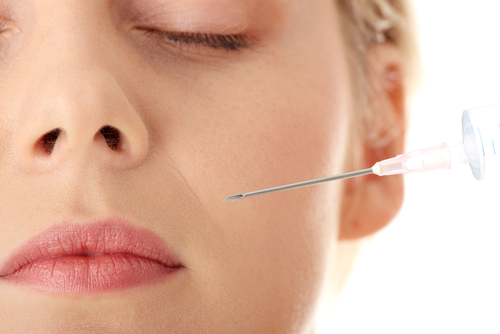 The FDA has approved a new dermal filler that contains enough lidocaine to provide nearly instantaneous numbing for patients getting facial wrinkles and folds filled.
The FDA has approved a new dermal filler that contains enough lidocaine to provide nearly instantaneous numbing for patients getting facial wrinkles and folds filled.
“Before the introduction of Juvederm XC, it often took up to 30 minutes for an anesthetic block to take effect,” clinical investigator Dr. Charles Boyd said in a press statement. The product has a similar safety profile as its predecessor, Juvederm, a gel hyaluronic acid dermal filler manufactured by Allergan.
The smooth consistency of the Juvederm gel makes it easier to inject and reportedly provides more natural looking results that last for up to a year. Injectable fillers provide a safe, non-surgical option for women and men who want to smooth facial wrinkles and folds without surgery, such as a brow lift or lower face lift.
The new formulation, Juvederm XC, maintains the same results as its predecessor and also contains a small amount of lidocaine, which numbs the treatment area within seconds, making the cosmetic treatment a more comfortable experience for the patient. The lidocaine is also intended to reduce or eliminate the need for any additional anesthetic.
The results during a double blind test were promising: 93 percent of patients reported less pain when treated with the new formulation of Juvederm compared to those treated with the non-lidocaine formulation.
Juvederm XC is now available nationwide; however, just like other dermal fillers, it should be administered by a trained medical professional.












Facebook
Twitter
Instagram
YouTube
RSS